COVID-19 Vaccine: Questions on Everyone’s Mind
Note: The original version of the article was published on December 16th in “The Daily Guardian”.

As of December 2020, worldwide around 57 vaccine trials are underway, with 17 trials in Phase 2 or 3. No vaccine trial has so far successfully completed a Phase 3 trial, although, several vaccines in Phase 3 have been approved for emergency use in various countries.
Let us break down testing terminology for you before we understand which vaccines are currently on their way to being approved? Phase 1 of testing usually marks the first time the vaccine is being tested in a very small group of adults (20–80 people), while in Phase 2 the study is expanded and given to people with characteristics similar to those for whom the vaccine is intended. In Phase 3, the vaccine is given out to thousands of people to test for its safety. Following Phase 3 (at times many go through Phase IV formal) a vaccine is approved and licensed by the respective issuing authority in the country.
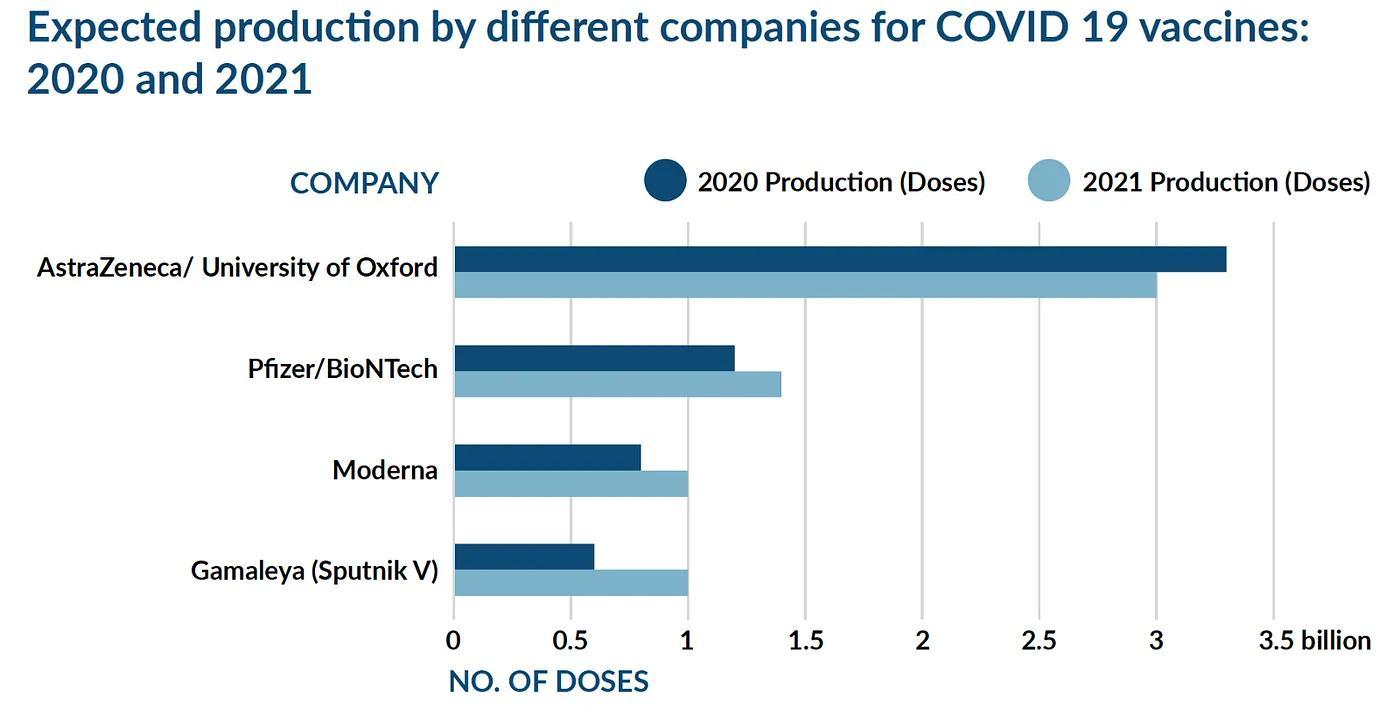
As of now, five major vaccine trials being undertaken by Pfizer, BioNTech, Moderna and the University of Oxford (along with AstraZeneca) and the Gamaleya Institute have announced positive results from their ongoing Phase 3 trials. At the same time, the Pfizer vaccine has already been approved for emergency use in the United Kingdom, United States, Bahrain, UAE and Canada. Pfizer and Moderna have both announced that they are attempting to maximize the availability of COVID-19 vaccines, and will produce around 50 and 20 million doses in 2020 respectively. In 2021, Pfizer is estimating that it will produce 1.3 billion doses and Moderna will produce between 500 million 1 billion doses. The current form of vaccination against COVID-19 is a set of two injections or doses administered a number of weeks apart.
What is the plan for distributing the COVID-19 vaccine in India?
Currently, three vaccines are under regulatory review in India, as announced by the head of the National Expert Group on Vaccine Administration for COVID-19 in early December. Three companies: Pfizer, Serum Institute of India (SII) and Bharat Biotech have applied to the drug regulatory body in India, the Central Drugs Standard Control Organisation (CDSCO), seeking approval for emergency use for viable candidates in India. Bharat Biotech’s Covaxin is currently undergoing Phase 3 trials in India, while SII has been conducting studies to bridge Covishield- developed by the Oxford University to India.
No vaccine has been approved for emergency use in India as of yet. However, the CEO of vaccine maker Serum Institute of India said Oxford University’s Covishield will be available for healthcare workers and other Phase 1 receipt by around February 2021.
Meanwhile, the Union Ministry of Health has also sent a set of operational guidelines to states and Union territories across the country about the process of distribution for the vaccine once it becomes available. So the question that now arises: when will you actually be able to get your hands on the COVID-19 vaccine? As per the guidelines issued by the UOH, in Phase 1, a total of 30 crore people will be given the vaccine.
The vaccine will be first distributed to health care workers (estimated population around 1 crore), followed by frontline workers (estimated population around 2 crores) and then to those who are above the age of 50 (estimated population around 26 crores). This will be followed by those below the age of 50 and suffering from a chronic critical illness (estimated population around 1 crore). The guidelines also detail that states/UTs can decide which dates they want to administer the vaccines and that only 100 people per “session” at each site per day will be allowed to be vaccinated. Following, this, the rest of the population (estimated population around 108 crores) will be given the Covid-19 vaccine, whenever it is ready for public use, based on the spread and availability of this disease. In order to identify people above the age of 50, the government will use the voter list prepared during the Lok Sabha and Assembly elections. The beneficiaries of the vaccine will be tracked in real-time through a digital platform called Co-WIN.
Health Secretary, Rajesh Bhushan announced this week that the Co-WIN mobile application is now downloadable and people can register themselves on it if they want to sign up for the vaccine.
Mass immunization: Indian companies planning to amp up delivery services
While the Indian government has announced the delivery of the vaccine to roughly around 30 crore people, the logistical delivery of the same will be a challenge for the country. Generally, vaccines need to be stored and transported between the temperatures of 2°C to 8°C, posing a key logistical hurdle in delivery. In order to maintain efficacy in the delivery of vaccines, it is necessary to have temperature-controlled transport and warehouses at ultra-low temperatures.
For several years after Independence, West Bengal had continued to be ruled by the INC. Although the CPI(M) emerged as the largest party in the state in the 1969 elections, after the Naxalbari uprising in1967 and the subsequent President’s rule declared in 1970, the Congress came back to power in West Bengal for one last time, under the leadership of Siddhartha Shankar Ray. Scholars and political analysts believe that the trend of using state resources to target opposition parties and members -which has become synonymous with politics in West Bengal — started with Ray’s regime. The 1972 elections were rife with electoral and political violence, and the Congress was accused of rigging the polls.
COVID-19 Vaccine: Free for all Indians?
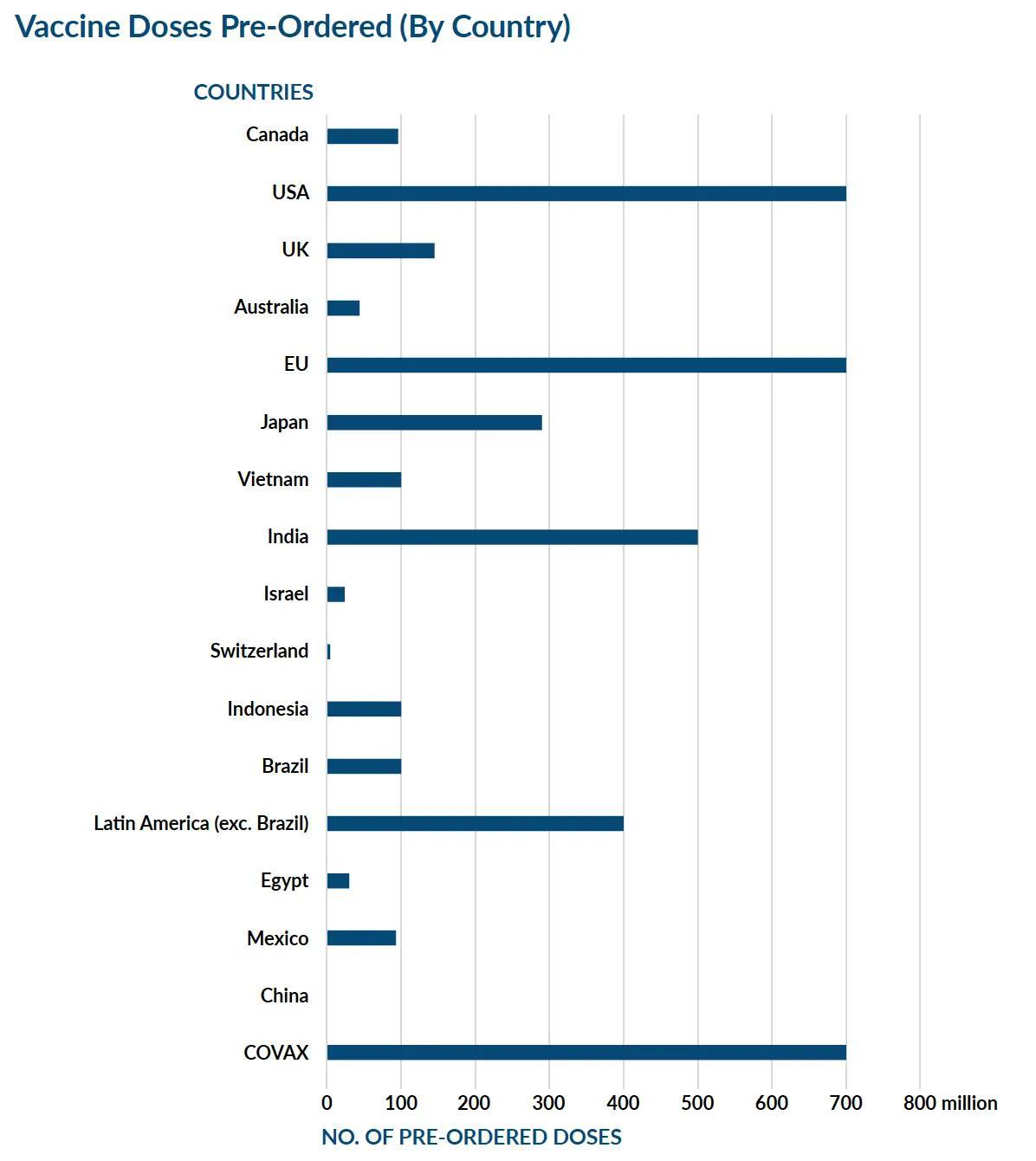
At a virtual conference in December, the Chairman and CEO of Pfizer, Albert Bourla revealed the tier wise pricing model being applied to price the Pfizer vaccine across the word. Speaking at the conference announced for International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), Bourla said developed countries would be charged for the vaccine on the basis of their GDP. Following this, a lower price would be charged for middle-income countries and low-income countries would be charged a price on a, not for profit basis. He also added that in developed countries such as the United States, the rates would remain very affordable.
In the United States, the price of the Pfizer vaccine to be given in two doses is estimated to be $19.50 (Rs. 1,434) per dose. Moderna also announced each dose of the vaccine will be for around $32–37 (Rs. 2,352 to Rs. 2,750). Each dose for Johnson & Johnson’s two-dose vaccine will cost around $10 (Rs. 735) and the University of Oxford and AstraZeneca’s two-dose vaccine is estimated to be the cheapest at around $3- $4 (Rs. 220–300) a dose. For India, vaccine maker Serum Institute of India has announced that the vaccine will be priced at a maximum of Rs. 1,000 for two necessary doses (Rs. 500 for each dose).
During the Bihar Assembly Elections in November, the Bharatiya Janata Party promised in its manifesto for the state that it would provide free COVID-19 vaccines for all. It also made the same promise in the manifesto for the Greater Hyderabad Municipal Corporation (GHMC) elections last week. Given such announcements, the question that is on everyone’s mind is: will Indians get the COVID-19 vaccine for free? Following the announcement by the BJP in Bihar, Union Minister Pratap Sarangi announced that all Indians will be given free COVID-19 vaccines. Additionally, the governments of Tamil Nadu, Madhya Pradesh, Assam and Puduchchery have also announced free of cost COVID-19 vaccines in their States.
Will Indians opt for a COVID-19 vaccine?
In November 2020, a survey carried out by Local Circles, a community social media platform revealed that Indians are likely to be hesitant of a COVID-19 vaccine. In the survey, 59% of all respondents (8,936 total respondents) said they will not rush to get the vaccine even if it is available by February 2021. Similarly, Accredited Social Health Activist (ASHA) workers conducting door to door survey about the vaccine in Gujrat earlier this month, said people are sceptical about being first in line for an “unknown vaccine”.
However, as per a global survey conducted by the World Economic Forum/IPSOS, 87% of Indians said they would get a vaccine for COVID-19. This figure was much higher than the global average, where overall, 73% of respondents said they would get a COVID-19 vaccine if available. The intent of vaccination amongst Indians has remained unchanged in 2 rounds of the surveys (August and November 2020). However, for 10 countries, including China, Australia, Spain, and Brazil, the intent of getting a vaccine amongst respondents have reduced.
What are the main reasons for not wanting to get a COVID-19 vaccine? Globally, the side effects are the highest cited concern (34%), followed about concerns about clinical trials moving too fast (33%). Similarly, 34% of Indians also said they are worried about the side-effects, while 16% said they are concerned about the fast-moving trials.
-Shreya Maskara/New Delhi
From Polstrat, a non-partisan political consultancy which aims to shift the narrative of political discourse in the country from a problem-centric to a solutions-oriented approach.
Read more about Polstrat here. Follow us on Medium to keep up to date with Indian politics.
